The Leahy-Smith America Invents Act provided for post issuance proceedings that allow the Patent Trial and Appeal Board (the PTAB) of the United States Patent and Trademark Office (USPTO) to review issued patents for validity. These proceedings have become a popular choice for defendants in patent litigation due to a lower standard for proving that a patent is invalid, the opportunity to participate in the proceedings, and a relatively shorter duration and lower costs when compared to litigation. One type of post issuance proceeding is an Inter Partes Review (IPR), in which a petitioner may challenge the validity of one or more claims of an issued patent under 35 U.S.C. §§ 102 or 103 in view of (only) patents or printed publications. The PTAB must grant the petition if the petitioner demonstrates that there is a “reasonable likelihood” that it can prove at least one claim is invalid. If the petition is granted, the PTAB is given one year to issue a final determination on the claims at issue.
On March 28, 2012, the Food and Drug Administration (FDA) published its first-ever guidance document on the risk-benefit analysis that forms the cornerstone of medical device approval.1 The 2012 guidance addressed considerations governing pre-market approval (PMA) and de novo submissions, but notably absent from the final version of the 2012 guidance was an explanation of the risk-benefit analysis for premarket notifications [510(k)]. On July 15th of this year, more than two years later, and amid a flurry of FDA guidance documents,2 the FDA released a draft guidance document specifically focused on the risk-benefit analysis for devices undergoing the 510(k) process. Although remarkably similar to the 2012 guidance, the 2014 guidance includes a handful of subtle differences that may be important for device manufacturers to keep in mind when making any type of submission.
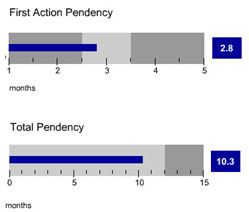 The articles in our earlier editions of the IP Bulletin addressed eight of the most commonly asked questions that arise when completing a U.S. trademark application filing. Once the application has been filed with the USPTO, substantive examination by an examining attorney from the USPTO will commence in due course. In stark contrast to the long waits experienced by patent applicants, the average time between application filing and the issuance of a first Office Action is only 2.8 months at the time of this publication. The average total pendency for a trademark application is only 10.3 months, which indicates that the application is either accepted or rejected by the examining attorney in less than a year.
The articles in our earlier editions of the IP Bulletin addressed eight of the most commonly asked questions that arise when completing a U.S. trademark application filing. Once the application has been filed with the USPTO, substantive examination by an examining attorney from the USPTO will commence in due course. In stark contrast to the long waits experienced by patent applicants, the average time between application filing and the issuance of a first Office Action is only 2.8 months at the time of this publication. The average total pendency for a trademark application is only 10.3 months, which indicates that the application is either accepted or rejected by the examining attorney in less than a year.
Currently, the average time a patent applicant waits to receive a first Office Action from the United States Patent and Trademark Office (USPTO) is 18.9 months. The total pendency for a patent application before a final disposition is achieved (e.g., Notice of Allowance issued, Request for Continued Examination filed, or application is abandoned) is 27.5 months. This number jumps to 37.9 months when applications in which RCEs are filed are included. These long delays can be frustrating for patent applicants. Start-up companies looking to secure patent protection to attract investors can find this long delay at the USPTO detrimental to the company’s ability to survive. Further, applicants looking to use information from U.S. patent prosecution to make decisions about which countries to enter following the filing of a Patent Cooperation Treaty (PCT) patent application may be left making expensive foreign filing decisions without much information about the likelihood of securing a patent.
On November 1, 2014, the European Patent Office (EPO) will implement a revision to Rule 164 EPC that allows Patent Cooperation Treaty (PCT) applicants to have more than one invention searched when a unity of invention objection is present, regardless of whether the EPO was selected as the International Searching Authority (ISA) during the international phase. Under current practice, if the EPO is not the selected ISA, a supplemental European search report will address only the first invention mentioned in the claims, and any further inventions can only be pursued through divisional practice. Conversely, however, if the EPO is the selected ISA, applicants can request and pay for additional searches before selecting the invention to be pursued.
Maximizing the protection and value of intellectual property assets is often the cornerstone of a business's success and even survival. In this blog, Nutter's Intellectual Property attorneys provide news updates and practical tips in patent portfolio development, IP litigation, trademarks, copyrights, trade secrets and licensing.